Nanoporous membranes are valuable tools for filtering impurities from water and many other applications. However, there is still much work to be done to perfect their designs.
Recently, Professor Amir Haji-Akbari’s laboratory demonstrated that the exact location of nanoscale holes on the membrane can make a big difference. The results are published in ACS Nano.
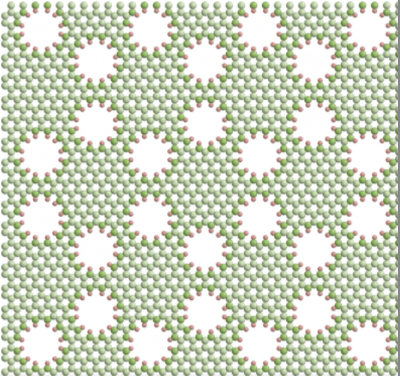
Nanoporous membrane. Image credit: Yale University
In recent years, nanoporous membranes based on graphene, polymers, silicon and other materials have been successfully used for gas separation, water desalination, virus filtration, power generation , gas storage and drug administration. However, creating membranes that let all the good molecules through while preventing the entry of unwanted molecules has proven tricky.
For water desalination, for example, the membrane must have high water permeability while sufficiently blocking small ionic and molecular solutes as well as other impurities. But researchers have found that increasing a membrane’s permeability often compromises its selectivity, and vice versa.
A promising approach is to optimize the chemistry and geometry of isolated nanopores to achieve the desired permeability and selectivity, and to place as many pores as possible in a nanoporous membrane. However, it is unclear how neighboring pores influence each other.
At the nanoscale, molecules interacting with pore walls can exhibit behaviors that defy conventional theories. The Haji-Akbari lab investigated whether it was possible to design innovative membrane systems with increased precision and efficiency by tuning nanopores.
Through computer simulations, Haji-Akbari’s research team discovered that the nanoscale proximity between pores can impair water permeability and salt rejection. Specifically, they created membrane simulations with different pore placement patterns, including a hexagonal lattice (figure above) and a honeycomb lattice (right). What they found was that the hexagonal pattern, which allowed a greater distance between pores, had higher permeability/selectivity performance than the honeycomb-patterned membrane.
These effects deviate from established theories, Haji-Akbari said.


A nanopore filter – artistic print. Image created by Alius Noreika using Copilot Designer
“This assumption that pore strength is independent of pore proximity is not correct,” said Haji-Akbari, assistant professor of chemical and environmental engineering. “Clearly, it depends on proximity. »
Their findings provide insight into how these effects accelerate the movement of some ions across membranes while causing other ions to decelerate. Additionally, it can contribute to better designs of nanoporous membranes for improved separation processes such as water desalination and other applications.
Source: Yale University
Originally published in The European Times.
source link eu news




















